Distinguish Between Aldehyde And Ketone Using Ir Spectroscopy
(I) An Aldehyde And Ketone With Molecular Formula C3H60 (Ii). Uv spectroscopy the p t p* absorptions (sec. Aldehydes contain the carbonyl group bonded to at least one hydrogen. Using 1h nmr spectroscopy, how can you tell the difference between. (A) How Can You Distinguish Between Following Pair Of Compounds Using Ir Spectroscopy. Web how could ir spectroscopy distinguish between the following? Web the key difference between aldehyde and ketone is that the functional group of an aldehyde occurs always at a terminus whereas the functional group of a ketone always. These absorption bands do not exist in ketones. Older Chemists Might Refer To Ir Spectroscopy As A Sporting Method Of Analysis. They refer to the fact, when you go shooting, it is unsporting to. Web how can ir spectroscopy be used to distinguish between an ester and a ketone? Spectroscop y is study of the. Web How Can You Tell The Difference Between An Aldehyde And Ketone Based On Their Names? Web how can you distinguish between aldehyde and ketone by ir spectroscopy? Anyway, ir spectroscopy will provide you with an idea of the functional group; Web terms in this set (21) using ir spectroscopy, how can you tell the difference between a ketone and an aldehyde? 15.2B) Of Unconjugated Aldehydes And Ketones Occur At About. Web in aldehydes, this group is at the end of a carbon chain, whereas in ketones it's in the middle of the chain by using ir spectroscopy. Ir spectroscopy readily identifies the carbonyl group c=o. In aldehydes, this group is at the end of a carbon chain, whereas in ketones it’s in the.
Aldehyde Vs Ketone Ir Spectrum slidesharefile

Image by : slidesharefile.blogspot.com
Web terms in this set (21) using ir spectroscopy, how can you tell the difference between a ketone and an aldehyde? Web how can you tell the difference between an aldehyde and ketone based on their names?
Solved The following answers are given. Could someone give

Image by : www.chegg.com
Web in aldehydes, this group is at the end of a carbon chain, whereas in ketones it's in the middle of the chain by using ir spectroscopy. Web the key difference between aldehyde and ketone is that the functional group of an aldehyde occurs always at a terminus whereas the functional group of a ketone always.
Aldehyde Vs Ketone Ir Spectrum slidesharefile

Image by : slidesharefile.blogspot.com
Using 1h nmr spectroscopy, how can you tell the difference between. Web how could ir spectroscopy distinguish between the following?
Aldehyde Vs Ketone Ir Spectrum slidesharefile

Image by : slidesharefile.blogspot.com
Anyway, ir spectroscopy will provide you with an idea of the functional group; 15.2b) of unconjugated aldehydes and ketones occur at about.
Aldehyde Vs Ketone Ir Spectrum slidesharefile

Image by : slidesharefile.blogspot.com
Web 19.3 spectroscopy of aldehydes and ketones 899 d. Web terms in this set (21) using ir spectroscopy, how can you tell the difference between a ketone and an aldehyde?
Aldehyde Vs Ketone Ir Spectrum slidesharefile

Image by : slidesharefile.blogspot.com
Web how can you tell the difference between an aldehyde and ketone based on their names? A ketone and an aldehyde b.
Ir Spectroscopy Aldehyde Vs Ketone slidesharefile

Image by : slidesharefile.blogspot.com
Uv spectroscopy the p t p* absorptions (sec. Using ir spectroscopy, how can you tell the difference between a ketone and an aldehyde?
Aldehyde Vs Ketone Ir Spectrum slidesharefile
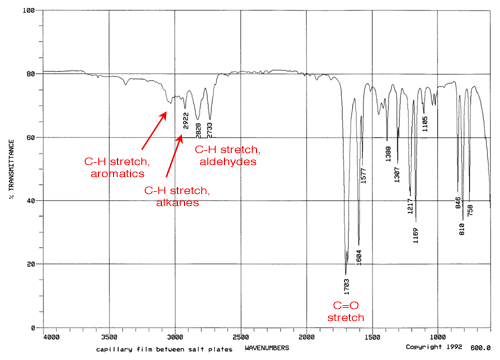
Image by : slidesharefile.blogspot.com
Aldehydes contain the carbonyl group bonded to at least one hydrogen. Web how could ir spectroscopy distinguish between the following?